Researchers around the world are collaborating to provide solutions to immediate and future challenges.
Scientists worldwide have united against a common enemy: COVID-19, the illness caused by the novel coronavirus SARS-CoV-2. Their aim? Slow and eventually stop the pandemic by joining forces and working rapidly on near-term and long-term solutions.
The following collection of images illustrates the range of ideas, from repurposing existing medicines to developing vaccines to searching for completely new ways to attack coronaviruses.
In the near term, researchers are focused on repurposing existing medicines. Existing medicines are available now and researchers already know a lot about how safe they are and how they work. Small studies in patients with COVID-19 suggest that some have the potential to help. But questions remain. Well-controlled, large-scale studies will help scientists determine the appropriate use of existing medicines to help COVID-19 patients.
In the long term, scientists aim to create new interventions that specifically address COVID-19. Making completely new medicines will take longer than repurposing existing drugs. Vaccines may take 12 to 18 months to be approved. Novel antivirals against coronaviruses could take years to discover and develop. If researchers are successful, however, COVID-19 could become a worry of the past.
Can we quiet an overactive immune system with existing medicines?
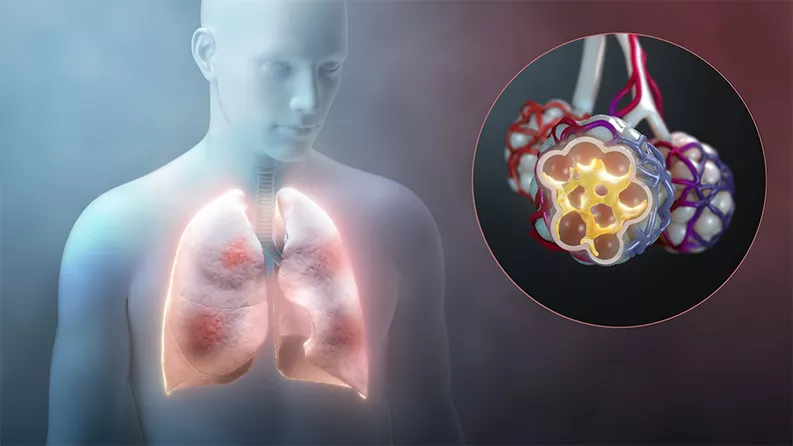
Many scientists and doctors want to know if drugs approved for use in other diseases – from cancer to arthritis and malaria – can help keep patients off ventilators and out of intensive care units.
Not everyone with COVID-19 has a severe reaction to the disease, but those who do may be experiencing an overreaction to the virus rather than damage from the virus itself. When the immune system recognizes the viral invader, it sends out a shower of signals that trigger immune cells to attack. In some cases – and for unknown reasons – that shower becomes a damaging storm that puts patients in jeopardy.
In the lungs, the overreaction gums up the walls of tiny air sacs. This buildup prevents oxygen from crossing into the bloodstream, and other organs can become oxygen starved. This condition is called acute respiratory distress syndrome. The lack of oxygen and the overreaction of the immune system can cause severe damage to the body.
Some existing drugs, however, act like an umbrella, blocking the rain of immune signals and slowing the runaway immune reaction. Novartis and other companies have several approved medicines for other diseases that work in this way or in a way that dampens the immune system. To determine if these drugs are safe and effective against COVID-19, scientists across the pharmaceutical industry are working with medical investigators to study the effects of these drugs on patients in controlled clinical trials.
In the near term, as trials begin and results emerge, these drugs have the potential to become safe and effective treatments for COVID-19 patients with serious illness. The availability of effective treatments could help the medical world meet the demands of the COVID-19 pandemic.
Can existing medicines block the virus from replicating?
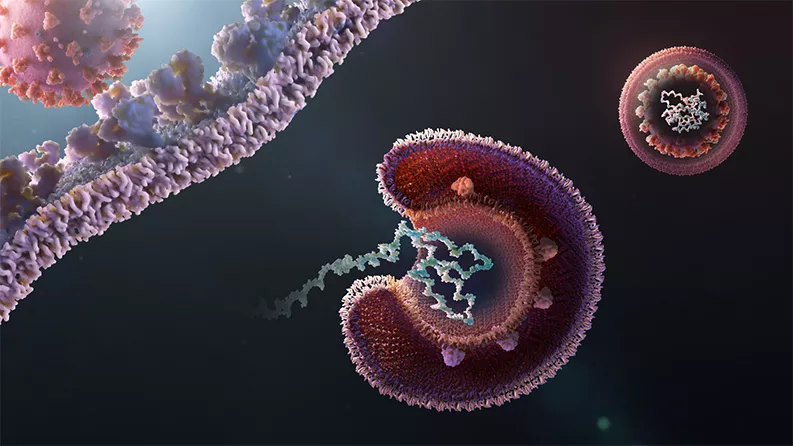
To cause an infection, a coronavirus first attaches to the outside of a human cell before penetrating the cell’s membranes. Once inside, the virus releases its contents and hijacks the cell’s machinery for creating large molecules called proteins.
That’s just the opening sequence. Like a TikTok dance, the steps get more complicated from there, including protein chopping, repetitive copying and more.
Existing medicines with so-called “antiviral properties” have the potential to interrupt different steps of this process. For instance, a drug might block the virus from entering a cell by preventing it from grabbing hold of the cell in the first place. Another drug might change the environment inside of cells and make it harder for the virus to release its contents.
Existing medicines were not designed with coronavirus in mind. And their structures were not optimized to interfere with the replication of COVID-19. But they might still have general antiviral effects that could help patients. The global scientific community is testing these ideas rigorously to learn more about how patients with COVID-19 respond to these medicines.
Novartis scientists, for example, are collaborating with medical investigators to support trials and donating medicines that have a scientific rationale suggesting they could help patients. Novartis is also launching trials of its own to learn more about which drugs work for whom and why. These trials could also help researchers understand when not to use an existing medicine, as individual differences can influence responses to a medicine.
Can scientists develop a vaccine to prevent infections?
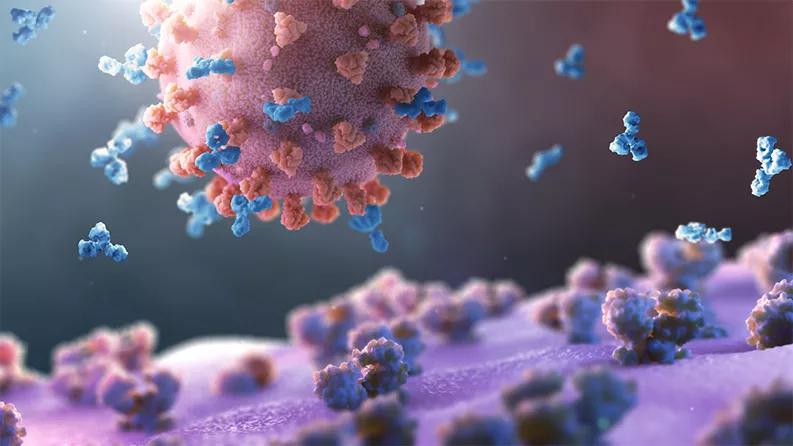
Several companies and academic institutions have started work on potential COVID-19 vaccines, which are given to people to protect them from infection.1 A few experimental vaccines have even entered clinical testing.
Vaccines present a virus or part of a virus to the body. The body’s immune system learns to recognize the virus and creates antibodies that attach to and disable invaders that look like it. Most attempts to create vaccines fail in humans even when they seem promising in the laboratory. It is important to have many approaches in development in parallel.
Scientists involved in vaccine development estimate that a COVID-19 vaccine could be developed and approved in 12 to 18 months. During that time, researchers will test vaccine candidates in small numbers of people to determine safety, then in larger groups to determine if the vaccine produces neutralizing antibodies. Researchers need to wait weeks or months after administering the vaccine before comparing the blood of vaccinated people and unvaccinated people to see if they have different immune responses to the virus. From there, if the vaccine has a positive effect, testing in a large number of people is required to ensure safety and a long-lasting immune response.
Scientists will also have to figure out how to manufacture the vaccine, how to distribute it, and whether to provide one-time shots or a series of boosters. If successful, the benefit will be substantial, but the complexities underscore the need for near-term antiviral options that are safe and effective.
Can researchers avert the next pandemic by discovering new medicines?
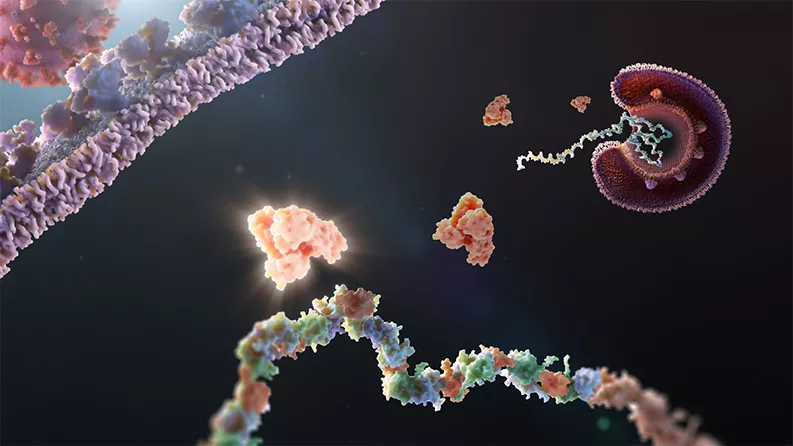
Coronaviruses have a few things in common. For one, under a microscope, they appear to have a crown (or “corona” in Latin) because of the spikes that protrude from its surface.
They also share an Achilles heel. These viruses, which cause SARS, MERS, some cases of the common cold, and COVID-19, all depend on a common piece of machinery. This machinery – a specialized set of molecular scissors called Mpro, or main protease – is essential for coronavirus replication.
The consistency of the scissors across coronaviruses makes it a prime drug target. In the years to come, another dangerous coronavirus will likely emerge. An antiviral that targets a shared feature of many forms of coronavirus could work against the next one.
Novartis researchers have teamed with other pharmaceutical scientists and academics on a quest to jam up these molecular scissors. The researchers are committed to open science, meaning that they are sharing ideas, resources and leads as they learn more. Even so, an effort to discover and develop a completely new medicine will likely take years. If successful, however, the world could be better prepared for the next coronavirus outbreak.