Media Library
icon
-
 PDFlockFact SheetCardiovascular disease – ORION-8 media summaryLong-term assessment of the efficacy and safety of inclisiran in patients with high risk of cardiovascular events
PDFlockFact SheetCardiovascular disease – ORION-8 media summaryLong-term assessment of the efficacy and safety of inclisiran in patients with high risk of cardiovascular events -
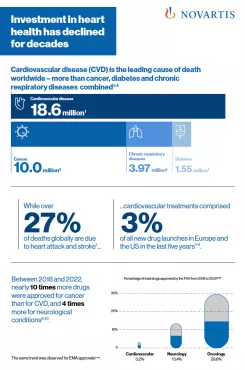 PDFlockFact SheetCardiovascular disease – By the NumbersCardiovascular disease (CVD) is the leading cause of death worldwide. Investment in heart health has declined for decades.
PDFlockFact SheetCardiovascular disease – By the NumbersCardiovascular disease (CVD) is the leading cause of death worldwide. Investment in heart health has declined for decades. -
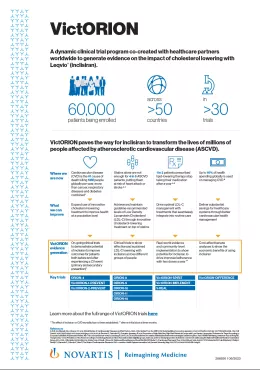 PDFlockFact SheetCardiovascular disease – VictORION media summaryA dynamic clinical trial program co-created with healthcare partners worldwide to generate evidence on the impact of cholesterol lowering with Leqvio® (Inclisiran).
PDFlockFact SheetCardiovascular disease – VictORION media summaryA dynamic clinical trial program co-created with healthcare partners worldwide to generate evidence on the impact of cholesterol lowering with Leqvio® (Inclisiran). -
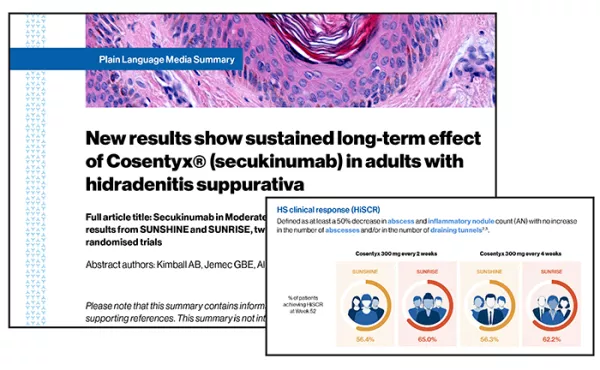 PDFlockFact Sheet52-week results for Cosentyx® in hidradenitis suppurativa: A summaryDiscover the data published in The Lancet on the long-term effect of Cosentyx® (secukinumab) in adults with hidradenitis suppurativa from the SUNRISE and SUNSHINE trials.
PDFlockFact Sheet52-week results for Cosentyx® in hidradenitis suppurativa: A summaryDiscover the data published in The Lancet on the long-term effect of Cosentyx® (secukinumab) in adults with hidradenitis suppurativa from the SUNRISE and SUNSHINE trials. -
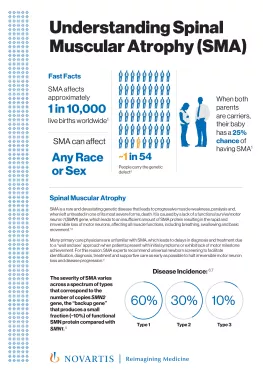 PDFlockDisease Awareness / Fact SheetSpinal Muscular AtrophyUnderstanding spinal muscular atrophy, a rare, neuromuscular genetic disease.
PDFlockDisease Awareness / Fact SheetSpinal Muscular AtrophyUnderstanding spinal muscular atrophy, a rare, neuromuscular genetic disease. -
 PDFlockScience & Innovation / Fact SheetThe Novartis AAV PlatformModified viruses – including adeno-associated viruses – are an efficient way to transport therapeutic genetic material to affected cells.
PDFlockScience & Innovation / Fact SheetThe Novartis AAV PlatformModified viruses – including adeno-associated viruses – are an efficient way to transport therapeutic genetic material to affected cells. -
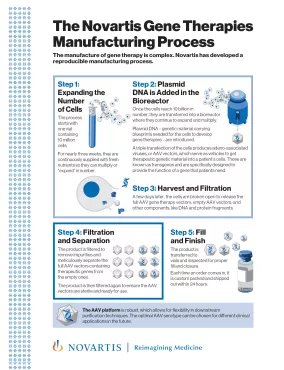 PDFlockScience & Innovation / Fact SheetThe Novartis Gene Therapies Manufacturing ProcessManufacturing of gene therapy is complex. Novartis has developed a reproducible manufacturing process.
PDFlockScience & Innovation / Fact SheetThe Novartis Gene Therapies Manufacturing ProcessManufacturing of gene therapy is complex. Novartis has developed a reproducible manufacturing process. -
 PDFlockCorporate Responsibility / Fact SheetAbout Novartis Gene TherapiesNovartis Gene Therapies is the world leader in gene therapy, and is reimagining medicine to transform the lives of patients.
PDFlockCorporate Responsibility / Fact SheetAbout Novartis Gene TherapiesNovartis Gene Therapies is the world leader in gene therapy, and is reimagining medicine to transform the lives of patients. -
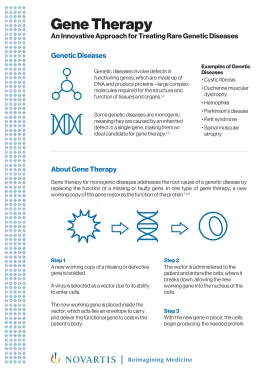 PDFlockScience & Innovation / Fact SheetAbout Gene TherapyGene therapy – an innovative approach to treating rare, genetic diseases.
PDFlockScience & Innovation / Fact SheetAbout Gene TherapyGene therapy – an innovative approach to treating rare, genetic diseases. -
PDFlockProducts / Fact SheetRTH258 Brolucizumab Clinical TrialsNovartis brolucizumab (RTH258) demonstrates superiority versus aflibercept in key secondary endpoint measures of disease activity in nAMD, a leading cause of blindness.
-
PDFlockProducts / Fact SheetKymriah® (tisagenlecleucel) in children and young adults with B-cell ALL that is refractory or relapsed at least twiceKymriah® (pronounced: Kim-RYE-ah) is the first FDA-approved CAR-T cell therapy available in the US
-
PDFlockProducts / Fact SheetThe JULIET Clinical TrialThe JULIET clinical trial is a global, multi-center Phase II registration trial investigating CTL019 (tisagenlecleucel) for use in diffuse large B-cell lymphoma (DLBCL), the most common form of non-Hodgkin lymphoma (NHL). The Novartis-sponsored JULIET trial was conducted in collaboration with the University of Pennsylvania to evaluate the safety and efficacy of CTL019 in adult patients with relapsed or refractory (r/r) DLBCL. Relapsed/refractory DLBCL is an aggressive (fast-growing), complex and difficult-to-treat disease, and patients with DLBCL often have worse prognosis than other forms of NHL.