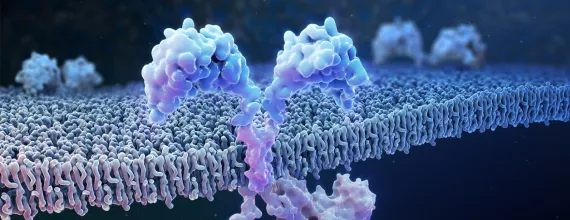
CAR-T cell therapy is currently one of Novartis' key focus areas for research and development
Individualized CAR (chimeric antigen receptor) T-cell therapy uses a patient’s own immune system to fight certain types of cancers. A patient’s T-cells are extracted and reprogrammed outside of the body to recognize and fight cancer cells and other cells expressing a particular antigen. CAR-T cell therapies are currently approved by various regulatory bodies to treat certain forms of advanced B-cell blood cancers. Novartis has a deep CAR-T pipeline. Our focus is to broaden the impact of cell therapy in oncology by going deeper in B-cell malignancies, potentially reaching patients in other hematological cancers and solid tumors and researching potential next-generation CAR-Ts that focus on new targets and utilize new technologies.