Duane Morgan can’t remember a time when he could see normally. Growing up in coastal Newfoundland, Canada, he avoided the dimly lit streets of his quiet hometown at night because his eyes couldn’t adjust to the dark. Then as a teenager, he developed a progressive and debilitating sensitivity to bright lights. Today, a glimpse of sunshine will blind him for hours.
"All I see is whitewash," says Morgan, who is now 42 and married with a 5-year-old daughter. "And when I turn away from the light, everything goes extremely dark."
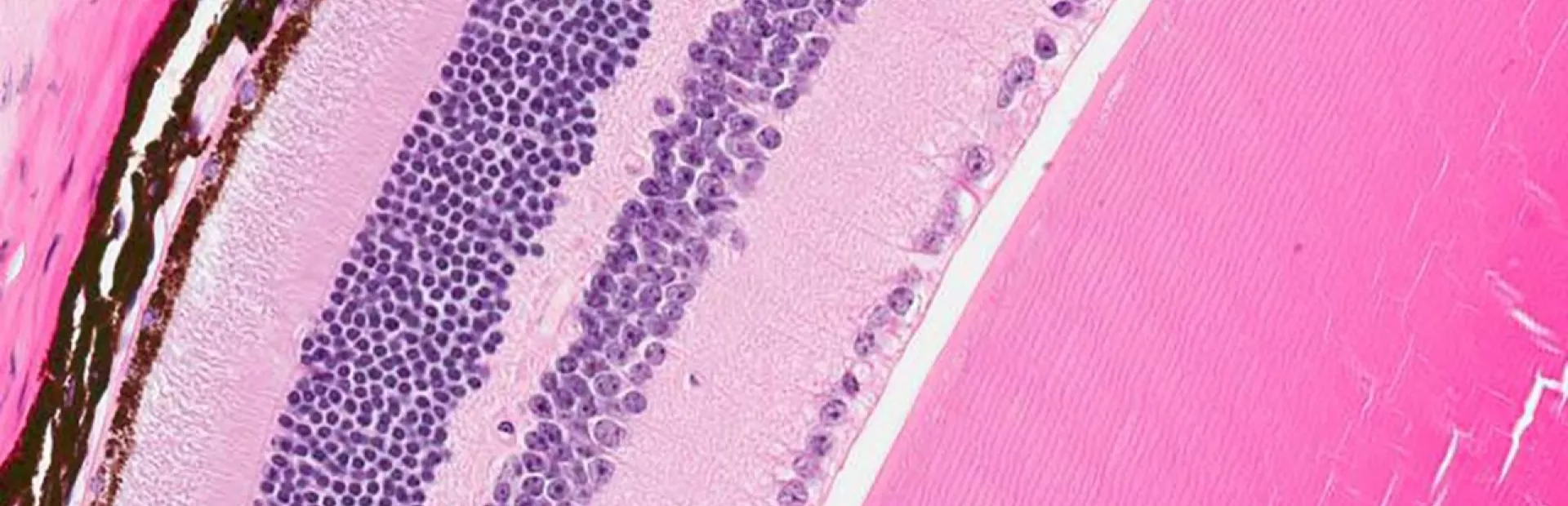
Morgan has retinitis pigmentosa (RP), which is a rare inherited disorder that cripples light-sensing cells in the retina (a layer of tissue in the back of the eye). Though it leads to visual problems in all patients, RP has varied genetic causes. In Morgan’s case, the culprit is a defective RLBP1 gene, which can’t make a crucial protein that light-sensing photoreceptor cells in the retina need to function properly. When deprived of that protein, the cells falter and eventually die, resulting in total blindness.
But an investigational gene therapy discovered and developed at the Novartis Institutes for BioMedical Research (NIBR) could potentially halt Morgan’s type of RP in its tracks. By inserting a normal RLBP1 gene into the relevant cells, Novartis researchers hope to fix the protein-making machinery and protect or even improve the patient’s vision. The investigational gene therapy is now being tested in a clinical trial in Sweden.
"RLBP1 RP has no approved treatment, making it a disorder with very high unmet medical need," says Kali Stasi, a Translational Medicine Expert in Ophthalmology at Novartis who leads the program and helped design the human study. "We’re aiming for a long-term successful impact on the patient’s visual function and quality of life."
The investigational study caps years of internal research at Novartis, and represents the company’s first effort toward producing a gene therapy from scratch. And as part of a broader Cell & Gene Therapy Initiative that spans multiple disease areas, it’s paving the way for future gene therapy programs that target other medical conditions.
Moving to the clinic
Several encouraging developments prompted the Novartis team to pursue the ongoing clinical trial.
Crucial evidence came from working with mice whose RLBP1 genes have been inactivated. Like humans with RP, these genetically modified animals adjust very slowly to the dark. After a single dose of gene therapy, however, their eyes recovered much faster from flashes of light, and the animals were still showing this visual function benefit a year after treatment.
"Gene therapy is promising because for some blinding diseases, it may offer the potential for a sustained benefit for patients," says Chad Bigelow, a Senior Investigator in Ophthalmology who designed the lab experiments.

Meanwhile, work by outside researchers showed that a gene therapy for another form of inherited retinal degeneration – caused by the mutation of both copies of the RPE65 gene – is clinically effective in people.1 This gave the team more confidence.
Before testing the investigational gene therapy in patients, the Novartis team had to address some key questions: What’s the best way to measure the treatment’s effects on visual function and quality of life? How does RP induced by RLBP1 mutations progress naturally when it’s not treated?
For answers, they teamed up with collaborators at Umeå University in Sweden and Memorial University of Newfoundland in Canada for an ongoing “natural history” study. That study enrolled 45 patients with the disease who agreed to be monitored for five years. Investigators assessed the kinds of routine challenges Morgan faces every day – for instance, difficulties with reading and navigating in the dark – and how these challenges worsen with time.
In 2017, at the annual meeting of the Association for Research in Vision and Ophthalmology, Novartis researchers working on the natural history study presented two years of follow-up data. This data will help the clinical trial team evaluate whether the gene therapy improves or stabilizes a patient’s ability to perform certain visual tasks.
A delicate procedure
The clinical trial is based at St. Erik Eye Hospital in Stockholm, Sweden. Anders Kvanta, a professor and retinal surgeon at the Stockholm-based Karolinska Institute, is leading the effort as principal investigator.
"It’s amazing to think we’re testing a potential treatment for what’s otherwise guaranteed blindness," says Kvanta. The safety and efficacy of this investigational gene therapy has yet to be established.
Individuals who enroll in the trial will first spend two weeks undergoing a battery of tests for baseline eye functioning. During the third week, they’ll receive a single gene therapy treatment given under general anesthesia to one eye only; the other eye will remain untreated. Kvanta will surgically remove gelatinous tissues behind the lens of the eye to gain access to the retina. Then he’ll inject a solution of specialized viruses – called adeno-associated viruses – designed in the lab to target the diseased retina cells. The viruses are engineered with safety and efficacy in mind, and they each carry a normal RLPB1 gene that will potentially churn out the needed protein.
Each participant will be treated only once. And if all goes as planned, the gene therapy might prevent the retinal tissues from degenerating further and potentially even improve visual function.
"This trial illustrates our commitment to bringing the most transformative and advanced medicines to the people who need them," says Melissa Liew, Head of Translational Medicine for Ophthalmology at Novartis. "The eye is a great target organ for gene therapy, providing a potential opportunity to significantly improve sight in patients with debilitating lifelong blinding diseases."
Meanwhile, Morgan says an effective treatment can’t come soon enough. "Now that I have a family and a young daughter, I’m getting more frustrated about the things I can’t do," he says. “I’d just like to be able to enjoy a sunny day and go outside to kick a ball with my kid. If I had one wish, it’s that gene therapy would cure my light sensitivity and allow me to keep whatever vision I have left."
Main image: Novartis researchers designed a gene therapy aimed at repairing the protein-making machinery of diseased retina cells (some shown in brown) to potentially preserve visual function in patients with a rare disease. Image by Oliver Turner/Novartis
Novartis has discovered an investigational #genetherapy that could treat a rare genetic #eyedisease.