In the 25 years he’s studied eye disease, Ganesh Prasanna – a Director of Ophthalmology at the Novartis Institutes for BioMedical Research (NIBR) – has seen big shifts in how doctors and scientists approach glaucoma. But it hasn’t been enough. Glaucoma remains the second leading cause of blindness in the world, and it affects more than 60 million people worldwide. We know it robs sight – beginning with peripheral vision – but we don’t know exactly why.
Glaucoma is associated with increased pressure in the eye. Medications that control this pressure can preserve vision for a time, but a number of patients on these drugs eventually go blind. This indicates that there are factors beyond pressure that drive the disease.
Following are excerpts from conversations with Prasanna about glaucoma research, the importance of understanding what happens in the eye and why it happens, and the possibility of future treatments.
Where did things stand when you first got into eye research?

Just before I got into the field, eye doctors started prescribing drugs called prostaglandins, the first drugs developed specifically for glaucoma. Until then, the best treatments were repurposed from heart disease, which also involves changes in pressure, just in the blood. Twenty years later, prostaglandins remain the standard of care for the most part. But they haven’t been the solution we’d hoped for. Patients with glaucoma are still going blind.
Why is that?
The biggest issue is that we are not tackling the roots of the disease. Today’s treatments go after the elevated eye pressure associated with glaucoma. But they don’t address the whole picture.
How do you mean?
We need to understand at a basic, cellular level what disease-related changes the eye experiences, and ask why they happen. What pathological changes occur within the cells that normally regulate fluid movement out of the eye, thereby increasing pressure in glaucoma? How do these cells control pressure in the first place?
And how, fundamentally, does pressure affect the retina? We know that retinal ganglion cells at the back of the eye that send visual information back to the brain through the optic nerve progressively die off in glaucoma patients, but we don’t know exactly why this happens.

What’s kept us from finding out?
A big obstacle has been a historic lack of human donor tissues available for study as well as consistent longitudinal assessments of the retinal structure and function to detect progressive loss. In the oncology field, you can take biopsies. You can sample tissues in the same patient over time to look for ongoing changes.
With glaucoma, you can’t do that. You can’t biopsy someone’s eye unless they’re having eye surgery. And for a long time people just didn’t know they could donate their eyes for research after they died. When people did donate, most often it was for cornea transplants. Once the cornea was collected, the rest of the eye was discarded.
What’s changed?
Very good relationships with eye banks have been key to the field as a whole. It’s increased access to patient samples, which has let researchers start to build complex models and simulate in the laboratory what happens to the eye. We’re learning just how complex and multifactorial glaucoma is.
So the field has a better starting point to work from now.

Yes. Now we can start doing fancy things like deep RNA sequencing, and compare which genes are on and off or elevated or decreased in cells from healthy eyes and diseased eyes. We can ask whether cells from glaucoma patients produce different profiles of proteins, lipids, pro- and antioxidants, etc., and see whether those factors cause eye pressure to go up or down, or affect the cells in the retina – specifically, retinal ganglion cells.
With these new capabilities, what do you want to discover?
From a drug discovery perspective, we’re focused on two things. First, we want to permanently transform fluid outflow in the eye to restore pressure to the levels seen in normal age-matched eyes, thus eliminating the need for sustained drug treatments. Second, we want to preserve the retinal ganglion cells that send visual information to the brain through the optic nerve. Scientists refer to this second approach as neuroprotection, and it’s particularly challenging. Imagine trying to measure how a drug will affect a slow degenerative process that takes years to play out.
You don’t sound daunted. Are you optimistic about finding transformative treatments or even cures?
Yes. We have a strategy for overcoming the challenge that I just mentioned. And we’ve recently laid the foundation for rational drug discovery in this field. Our scientists can finally attempt to target the roots of glaucoma because we’ve started to identify them through external collaborations with cutting-edge researchers. In addition, we have exciting new tools and technologies – from imaging platforms to single-cell RNA sequencing – at our fingertips. There’s no guarantee of success in science, but we’ve got momentum. This is going to be fun.
A version of this Q&A was originally published in 2014. It has been updated to reflect research progress.
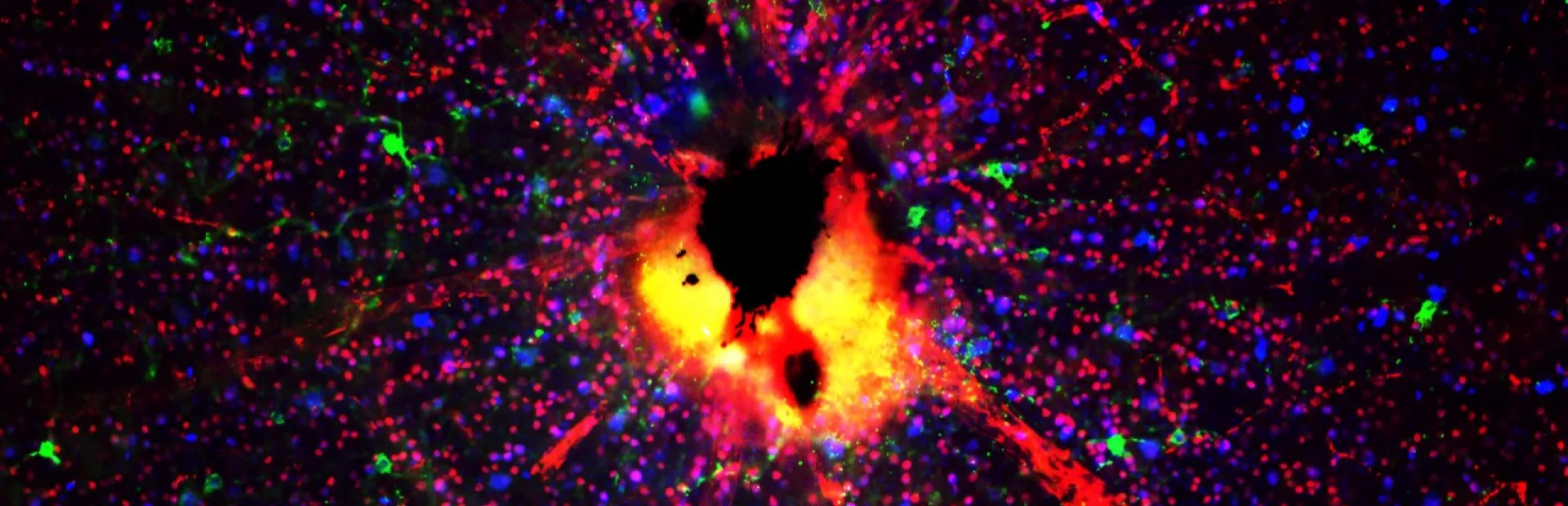
Main image: Researchers are building models with retinal ganglion cells (shown here as red and green dots) to learn more about the molecular mechanisms of glaucoma. The cells in this image are from mice. Image: Chenying Guo, Novartis.
Novartis scientist Ganesh Prasanna discusses #glaucoma research.